MEMBRANE TRANSPORT [CSEC BIOLOGY & HSB]
SYLLABUS REFERENCE
HSB
[A6] explain the importance of passive and active transport in living systems;
CSEC BIOLOGY
- [B1.6] explain the processes of diffusion and osmosis;
- [B1.7] discuss the importance of diffusion, osmosis, and active transport in living systems;
WHAT IS MOLECULAR TRANSPORT?
Molecular transport is the transfer of particles form one point to another. In living systems, these particles tend to be molecules or ions.
This is possible since all matter is made up of particles, according to the Particulate Theory of Matter.
The particles usually move between spaces that differ in their concentration.
COMPARING THE TYPES OF PASSIVE TRANSPORT
DIFFUSION
Definition
The movement of particles from an area where it is in high concentration to an area of lower concentration.
Since diffusion does not require a semi-permeable membrane, it can happen anywhere there is a difference in concentration.
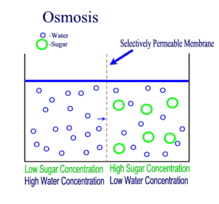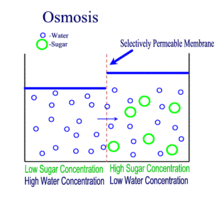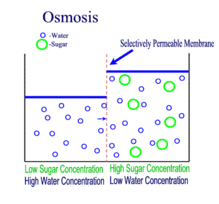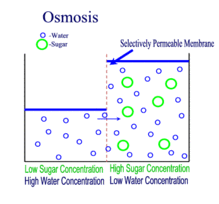
OSMOSIS
Definition
The movement of water molecules from an area where it is in high concentration to where there is a lower concentration, across a semi-permeable membrane.
One can also say...
Osmosis is the movement of water molecules from a dilute to concentrated solution, separated by a semi-permeable membrane.
Note:
In a dilute solution, the substance dissolved in water (for example, salt) is in low concentration. At the same time, however, the water in the dilute solution is in high concentration.
This is why water would move down its own concentration gradient from a dilute solution to a concentrated solution.
A partially / semi-permeable membrane is necessary for osmosis to occur. The membrane 'selects' for the very small water molecules, and blocks the larger molecules from crossing it.
Since a cell membrane is a semi-permeable membrane, it facilitates osmosis between a cell and its surroundings.
ACTIVE TRANSPORT
Definition
This is the movement of ions or molecules in or out of a cell against their concentration gradient, using energy released by respiration.
It occurs only in living systems, since it requires a lot of energy, obtained via respiration.
It is facilitated by a carrier protein that is located within the cell membrane, using the following steps:
- The molecule/ion combines with a carrier protein.
- Respiration provides the energy (in the form of ATP) for the carrier protein to change its shape.
- With the change in shape, the molecule/ion is moved to the other side of the cell membrane.
- The carrier protein releases the molecule/ion and changes back to its original shape.
 |
| ACTIVE TRANSPORT OF SODIUM & POTASSIUM ACROSS THE CELL MEMBRANE |
MOLECULAR TRANSPORT IN LIVING SYSTEMS
DIFFUSION AND OSMOSIS
Gaseous Exchange at the Lungs
The lung tissue is arranged into clusters of alveoli (tiny air sacs), each of which has ideal conditions for rapid diffusion, including a very thin membrane and a rich blood supply.
OSMOSIS EFFECTS ON PLANT VS ANIMAL CELLS
A cell is basically some chemicals in dissolved in water, inside a partially permeable membrane. The cell contents make a concentrated solution, and therefore will tend to draw water into it via osmosis.
- Marine invertebrates such as jellyfish. Osmosis causes no issues since the concentration of their cells exactly match that of the surrounding sea water. Therefore, there is not net movement of water into or out of the cells.
- Freshwater fish. They have a major water balance problem. The freshwater constantly flows over the gills so that oxygen can be extracted for respiration. However, at the same time, water gets into the gill cells and blood via osmosis. If allowed to continue undisturbed, this threatens to produce hypotonic conditions in the organism's fluids, such as blood plasma. To prevent this, excess water is removed via the production of large quantities of dilute urine. They also have special salt-absorbing glands that pump salt into the cells via active transport.
- What happens to red blood cells when drops of blood are placed in different solutions?
- Hypotonic solution (concentration is lower than that of blood plasma): the water goes red, but it is not cloudy. There are no red blood cell observed when examined under a microscope. Water has moved via osmosis into each red blood cell. So much water has entered that the membranes have burst.
- Isotonic solution (concentration matches that of blood plasma): the liquid becomes cloudy red. Normal red blood cells are observed under a microscope. No concentration gradient, so no water moves into or out of the red blood cells.
- Hypertonic solution (concentration higher than that of blood plasma: The cells appear crinkly under a microscope. Water has left the cells via osmosis, and cell volume has decreased.
- Plant cells respond well to hypotonic conditions. The cells fill up with water and the cell wall prevents bursting. The result is a very firm (aka 'turgid') cell. Turgid cells are very important to plant support, especially in non-woody plants.
- In hypertonic conditions, the cells can lose water and shrink to the point where the cell membrane tears away from the cell wall, killing the cell.